Biotech policy is getting attention on the Hill
Biotech policy: Recent policy discussions echo NSCEB recommendations across investment, defense, and data.
As I’m working towards building a part of my research program in AI and biosecurity (partially in collaboration with my colleagues at UVA’s National Security Data and Policy Institute), much of the work in AIxBio intersects with policy. I’ll occasionally write about that intersection here (note, policy ≠ politics; don’t come here expecting any political commentary).
Lately there’s a lot happening in the biotech space on Capitol Hill. NSCEB has effectively turned “biotech is strategic” from a slogan into a legislative checklist. Expect more authorizing pushes in 2026, but also keep an eye on the appropriations side, because that is where “policy intent” becomes real capacity.
NSCEB report and paper
If you have been watching U.S. biotech policy drift around in “important but vague” territory, the last few months have felt different. A big driver is the National Security Commission on Emerging Biotechnology (NSCEB), whose final report (released April 2025) laid out a federal action agenda spanning innovation, industrial capacity, security tools, and international coordination.
(By the way, check out a series of posts my my friend, colleague, and NSCEB commissioner, Alexander Titus, on the NSCEB report: part 1, 2, 3, 4, 5, and 6.)
NSCEB is also feeding the conversation with follow-on work. A late-November discussion paper, The Future of Science: A Playbook for Accelerating American Innovation, argues for updating how the federal government partners with universities, industry, and philanthropy, and for building the conditions for more automated and distributed scientific discovery.
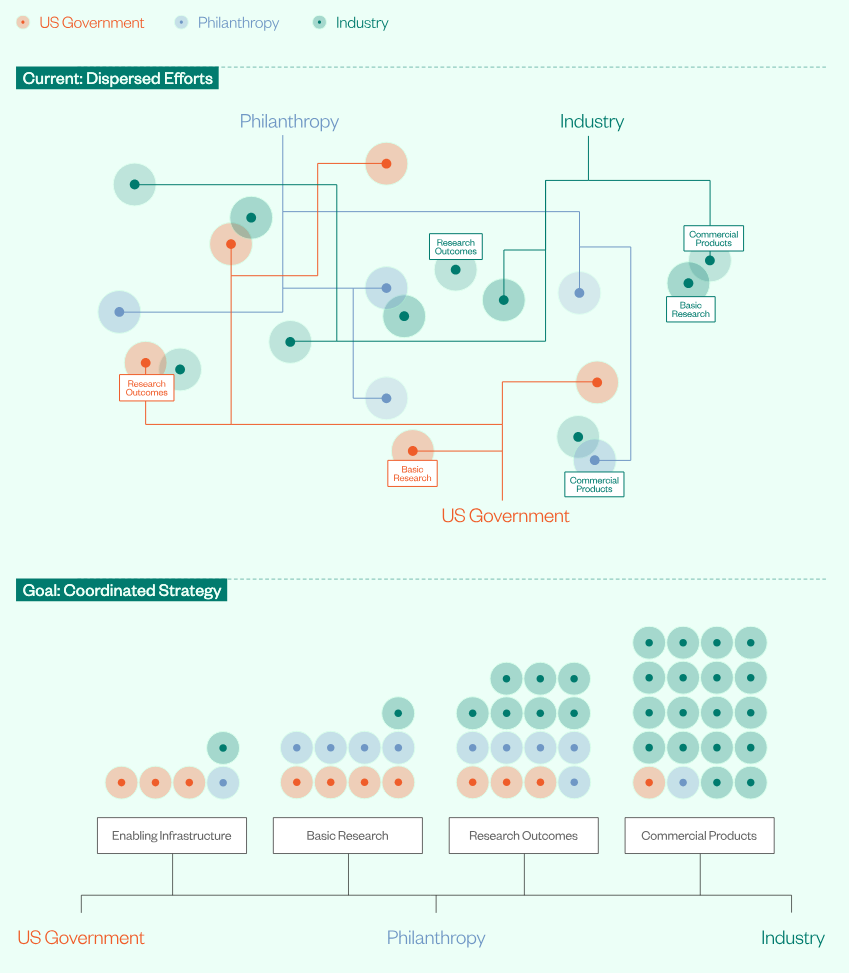
Two House bills map cleanly onto the “lab-to-market” and “defense strategy” lanes
One visible throughline is that members of the Congressional BIOTech Caucus are translating parts of the NSCEB playbook into authorizing legislation.
An investment-fund approach to the “valley of death.” Rep. Chrissy Houlahan and Rep. Pete Sessions introduced the Independence Investment Fund Act of 2025 (H.R. 6412), which would stand up an independent fund inside Treasury to make equity-style investments intended to pull critical and emerging technologies, including biotech, closer to scale and commercialization.
A defense strategy mandate for emerging biotechnologies. The pair also introduced the Defense Biotechnology Strategy Act (H.R. 6009), directing DoW to develop a strategy focused on the national security implications of emerging biotechnologies.
The FY2026 NDAA is also carrying some biotech content
Even when standalone bills stall, the National Defense Authorization Act is historically one of the most reliable “must-move” vehicles for defense-adjacent tech priorities.
This year’s conference text explicitly includes a dedicated slice of RDT&E language labeled “Biotechnology Matters” (Subtitle C, page 102). Some highlights:
Build capacity, not just prototypes: The bill language authorizes RDT&E support that can extend into designing and constructing bioindustrial manufacturing capabilities, reflecting a clear “scale it domestically” posture.
Create a governance home inside DoD: It establishes a senior biotech lead and a Biotechnology Management Office intended to coordinate development, acquisition, and sustainment of biotech capabilities across the department.
Turn biomanufacturing into a commercialization pathway: Multiple provisions are framed to incentivize private-sector infrastructure expansion and to support transition from military labs to operational capability, which is another “bench-to-field” bridge.
Treat biological data as AI-relevant infrastructure: The NDAA materials also point to requirements around collection and storage of biological data resources generated with DoD funding so they are usable for AI-enabled analysis, which is a subtle but important “data plumbing” move.
Put guardrails and geopolitical constraints in writing: There are provisions aimed at ethical/responsible development and deployment, procurement preferences for biobased products that meet defense needs, and restrictions related to providers of concern/foreign adversaries, plus reporting on China’s biotech advances.
The House passed the FY2026 NDAA conference bill on December 10, 2025, and the Senate is expected to take it up this week. Practical dollars for FY2026 defense priorities still hinge on appropriations decisions and timing. We’ll see what happens over the next month or so.


